Abstract
Background Treatment of acute myeloid leukemia (AML) leads to significant healthcare resource utilization (HRU), including extended hospitalization and transfusions. Standard treatment regimens include the high intensity "7+3” (anthracycline + cytosine arabinoside) and CPX-351 (daunorubicin + cytarabine liposome) used in younger, fit patients, and the lower intensity hypomethylating agent + venetoclax (HMA-Ven) used in patients deemed ineligible for intensive induction. Previous reports show comparable remission rates between regimens. Studies have shown no significant difference in HRU between 7+3 and CPX-351, but there have yet to be reports on HRU for HMA-Ven compared to high intensity regimens.
Methods We retrospectively analyzed patients with newly diagnosed AML treated at UC Davis Medical Center from 5/2012 to 5/2021 with HMA-Ven, 7+3, or CPX-351, identified using pharmacy and EMR reports. Patients met criteria if they achieved first complete remission with (CR) or without (CRi) count recovery as per ELN 2017 criteria. The date of CR1 was defined as date of first bone marrow biopsy following induction with evidence of CR/CRi. Primary outcomes assessed were number of packed red blood cell (pRBC) units transfused, platelet (Plt) units transfused, and hospital days (HDs) from start of treatment to CR1. Secondary outcomes assessed were HRU in 60 and 90 days post-CR1, time to count recovery, and time to CR/CRi. Survival outcomes included relapse-free survival (RFS), overall survival (OS), and estimated 12- and 24-month survival. Data were analyzed with SPSS; pairwise comparisons of medians were performed using Wilcox Sum Rank test, and time to event analyses were performed via the Kaplan-Meier method.
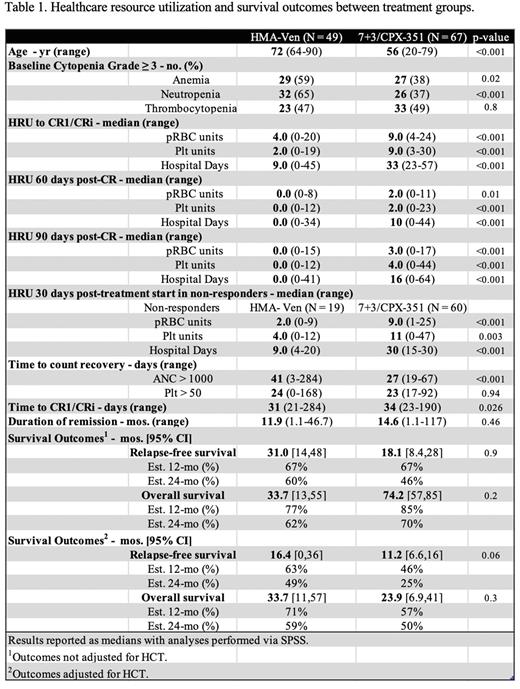
Results We identified 68 patients treated with HMA-Ven, 49 of which achieved CR/CRi (53% CR, 19% CRi). 60 of 113 patients (53%) treated with 7+3 achieved CR1 without re-induction, as well as 7 of 14 patients (50%) treated with CPX-351. No patients were enrolled in clinical trials. Of the 7+3 patients who achieved CR1, 14 received additional agents: midostaurin or gemtuzumab. Initial analyses revealed no significant difference between 7+3 and CPX-351 patients in all three primary HRU outcomes (p>0.05). Thus, those patients were analyzed as a single high-intensity treatment group and compared with the lower-intensity HMA-Ven group. Median age for HMA-Ven patients was 72 years, significantly higher than 56 years for the 7+3/CPX group (p<0.001, Table 1).
The median days to CR1/CRi was 31 days for the HMA-Ven group and 34 days for the high-intensity group. Patients treated with HMA-Ven required significantly fewer pRBC and Plt units and hospital days than the 7+3/CPX group from start of treatment to CR1 (p<0.001, Table 1). At 60 and 90 days post-CR1, these results were maintained (p≤0.01). Similar results for all three primary outcomes were seen amongst the non-responders of each group with an endpoint of 30 days post-treatment start (p<0.01).
Of the HMA-Ven and 7+3/CPX patients, 24% and 63%, respectively, proceeded to HCT. The high-intensity patients who did not receive HCT were placed on midostaurin maintenance or surveillance. There were no significant differences between survival outcomes before adjusting for HCT (p>0.05). The median RFS was 31 months [95% confidence interval (CI), 14-48] for HMA-Ven and 18.1 months [CI, 8.4-28] for 7+3/CPX. Median OS was 33.7 months [CI, 13-55] for HMA-Ven and 74.2 months [CI, 57-85] for 7+3/CPX. Estimated 12- and 24-month RFS was 67% and 60% respectively for HMA-Ven and 67% and 46% for 7+3/CPX. Estimated 12- and 24-month OS was 77% and 62% respectively for the HMA-Ven group and 85% and 70% for 7+3/CPX. After adjusting for HCT, no significant difference was also seen (p>0.05).
Conclusion To our knowledge, this is the first assessment of HRU in AML patients treated with HMA-Ven. We found that they required significantly fewer pRBC and Plt transfusions and significantly shorter hospitalizations compared with 7+3 or CPX-351 treated patients, despite a significantly higher median age. These differences were maintained 60- and 90-days post-CR. RFS and OS were not statistically significantly different between treatment regimens. Limitations include the retrospective and single center study design. Our data suggest that HRU should be prospectively evaluated in AML and may be relevant to consider when recommending treatment options.
Disclosures
Beechinor:National Institute of Health: Consultancy, Research Funding; Eunice Kennedy Shriver National Institute for Child Health and Human Development: Consultancy, Research Funding; Children's Oncology Group: Consultancy, Research Funding; IQVIA: Consultancy, Research Funding; Pfizer, Inc.: Consultancy, Research Funding; Trinity Life Sciences: Consultancy, Research Funding; Oncology Reimbursement Management: Consultancy, Research Funding; Aptitude Health: Consultancy, Research Funding; The Dedham Group: Consultancy, Research Funding; Cempra Pharmaceutics: Consultancy, Research Funding. Rosenberg:Adaptive: Consultancy; Bristol Myers Squib: Research Funding; Kangpu: Other: Institutional Research; Takeda: Other: Institutional Research; Janssen, Takeda: Speakers Bureau. Hoeg:Orca Bio: Research Funding. Tuscano:Celgene: Research Funding; Genentech: Research Funding; Pharmacyclics: Research Funding; Takeda: Research Funding; Achrotech: Research Funding; ADC therapeutics: Research Funding; BMS: Research Funding. Jonas:Gilead: Consultancy, Other: data monitoring committee , Research Funding; Pfizer: Consultancy, Research Funding; AbbVie: Consultancy, Other: Travel Reimbursement, Research Funding; GlycoMimetics: Consultancy, Other: protocol steering committee , Research Funding; Jazz: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; BMS: Consultancy, Research Funding; 47: Research Funding; Servier: Consultancy; Takeda: Consultancy; Tolero: Consultancy; Treadwell: Consultancy; Accelerated Medical Diagnostics: Research Funding; Amgen: Research Funding; AROG: Research Funding; BMS: Consultancy, Research Funding; Celgene: Research Funding; Daiichi Sankyo: Research Funding; F. Hoffmann-La Roche: Research Funding; Forma: Research Funding; Roche: Research Funding; Hanmi: Research Funding; Immune-Onc: Research Funding; Incyte: Research Funding; Loxo Oncology: Research Funding; LP Therapeutics: Research Funding; Pharmacyclics: Research Funding; Sigma Tau: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.